The Nitrogen Lone Pair of This Molecule Is Delocalized.
The carbon directly attached to the functional group in an organic molecule is referred to as the alpha carbon and the hydrogen attached to an. And since we know that that lone pair is de-localized its going to occupy a P orbital and so therefore this nitrogen is SP two hybridized because we know SP two hybridization has three SP two hybrid orbitals and one P orbital.
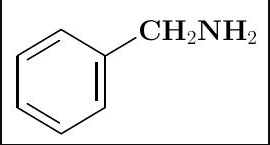
Solved The Nitrogen Lone Pair Of This Molecule Is Chegg Com
Since the sp2 orbital is orthogonal to the p orbital the lone pair is not able to overlap with the delocalized p orbitals from the ring carbons.

. The effect is seen in certain amines phosphines sulfonium and oxonium ions sulfoxides and even carbanions. Up to 256 cash back The lone pairs on oxygen in the following are compound are ____ A both in sp3 orbitals and both are localized B both are in p orbitals and both are delocalized C one is in p orbital and delocalized and the other is in sp2 orbital and localized D one is in p orbital and delocalized and the other is in sp3 orbital and localized E one in sp2. The resolution of enantiomers where the stereogenic center is an amine is usually precluded because the energy barrier for nitrogen.
Organic Chemistry Hybridization and Atomic and Molecular Orbitals Molecular Orbitals and Hybridizations 1 Answer Truong-Son N. Delocalized electrons and resonance 11 pts total Circle any of the following compounds that have a nitrogen atom with delocalized electrons. As always the organic chemist exploits reaction conditions that select a particular reactivity.
The nitrogen atoms are bonded with triple bonds. The nitrogen lone pair in pyrrole sits in a p orbital which makes it available for overlap with the p orbitals of the ring carbons. Quinoline is a base because the lone pair of electrons on the nitrogen atom of pyridine is not involved in the formation of a delocalized π -molecular orbital.
258 Each nitrogen atom has a lone pair that is delocalized via resonance In from CHEM 2070 at Auburn University. Label each lone pair in the molecule below as localized L or delocalized DL. Not to my knowledge.
QUESTION 5 The nitrogen lone pair of this molecule is delocalized. Only compounds where the nitrogen lone pair is directly attached to a pi bond. 213 Delocalized and Localized Lone Pairs True or false.
Solution for The lone pair on the nitrogen atom below is delocalized. This problem has been solved. Hence total number of lone pair is 2.
NEÖH a What is the hybridization and molecular geometry of. The nitrogen lone pair of this molecule is delocalizedTRUE OR FALSE. So each of them have a lone pair of electron.
Start your trial now. The nitrogen lone pair of this molecule is delocalizedTRUE OR FALSE. The difference between localised and delocalised chemical bonds is that a localised chemical bond is a specific bond or a lone electron pair on a specific atom whereas a delocalised chemical bond is a specific bond that is not associated with a single atom or a covalent bond.
Both molecules are aromatic but the nitrogens lone pair sticks out in pyrridine and is delocalized as part of the pi-ring in pyrrole. It is true because lone View the full answer Transcribed image text. Correct option is A Correct answer.
Could potentially bind thru either atom in the molecule. Could darth plagueis beat palpatine February 3 2022 delocalized lone pair of electrons 0 Comment February 3 2022 delocalized lone pair of electrons 0 Comment. The nitrogen atom in the molecule shown below has a delocalized lone pair of electrons.
The lone pair is localized on the nitrogen atom and this is the nucleophilic centre. We gots H 3N. On the other hand the nitro group N O 2 or C N.
Ive read half a dosen discriptions online but am clearly missing something. As always the organic chemist exploits reaction conditions that select a particular reactivity. If a substituent has a lone pair on the atom directly attached to a benzene ring then the lone pair can be delocalized into the ring electron withdrawal by resonance if a substituent is attached to a benzene ring by an atom that is doubly or triply bonded to a more electronegative atom then the electrons of the ring can be delocalized onto the substituent.
NH2 O True O False close. The nitrogen lone pair in pyridine sits in an sp2 orbital. Reason- Molecular formula of Nitrogen molecule is N2.
We gots the lone pair is localized on the nitrogen atom and this is the nucleophilic centre. Hydrogen-bonding forces between molecules are stronger than dipole-dipole interactions so molecule A has a higher boiling point. The lone pair on the nitrogen atom below is delocalized.
Solve any question of Chemical Bonding and Molecular Structure with-. In which structure does nitrogen bear a positive formal charge. Quinoline is aromatic with a resonance energy of 473 and is considered to be a resonance hybrid of the following contributing structures Figure A.
On the other hand the nitro group could potentially bind thru either atom in the molecule. NH2 True O False. A lone pair can contribute to the existence of chirality in a molecule when three other groups attached to an atom all differ.
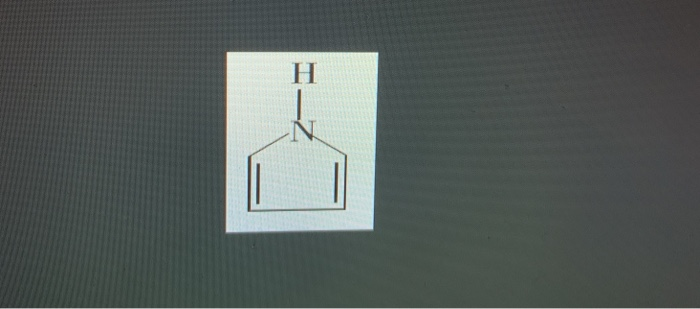
Solved Question 5 The Nitrogen Lone Pair Of This Molecule Is Chegg Com
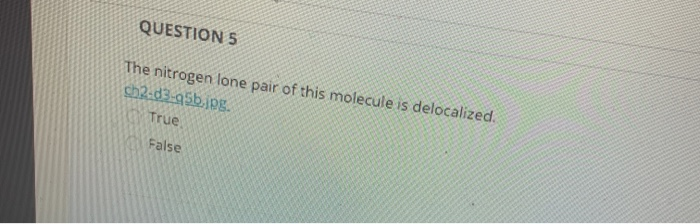
Solved Question 5 The Nitrogen Lone Pair Of This Molecule Is Chegg Com

Solved The Nitrogen Lone Pair Of This Molecule Is Chegg Com
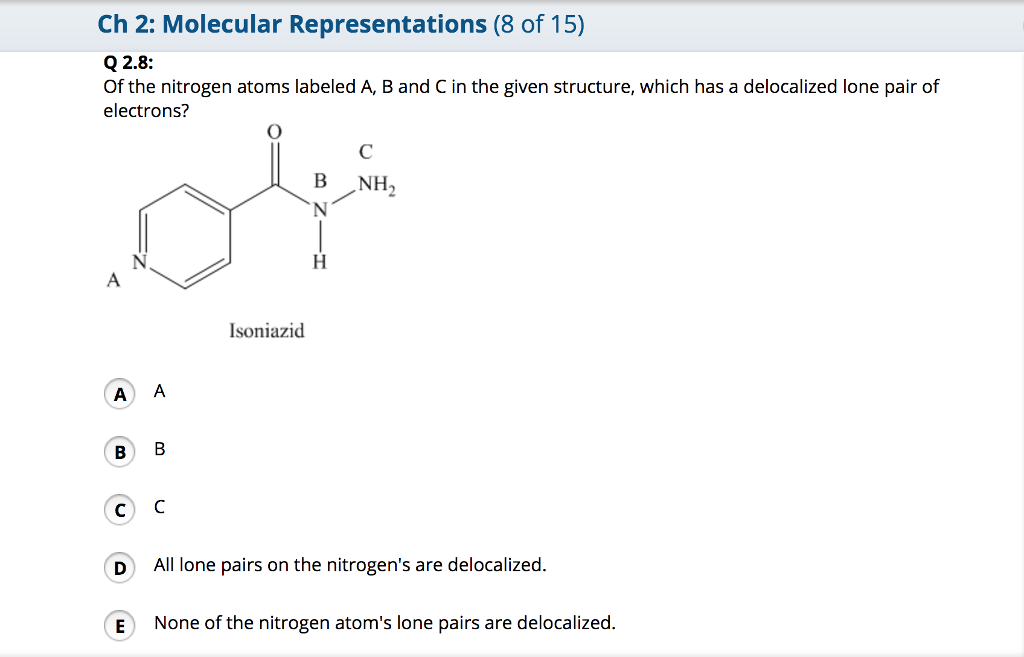
Solved Of The Nitrogen Atoms Labeled A B And C In The Given Chegg Com
No comments for "The Nitrogen Lone Pair of This Molecule Is Delocalized."
Post a Comment